Quasi-static processes
The special symbols
| (125) |
Hence, if the external parameters are changed by infinitesimal amounts, so that
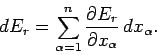 | (126) |
The work
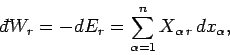 | (127) |
where
| (128) |
is termed the generalized force (conjugate to the external parameter
where
is the mean generalized force conjugate to
| (131) |
where
| (132) |
is the infinitesimal volume change due to the displacement of the surface. It follows from (130) that
| (133) |
so the mean pressure is the generalized force conjugate to the volume
| (134) |
This quantity is just the ``area under the curve'' in a plot of
It is important to distinguish between reversible and quasistatic processes. Reversible processes are always quasistatic, but the converse is not always true. For example, an infinitesimal compression of a gas in a cylinder where there exists friction between the piston and the cylinder is a quasistatic process, but not reversible process. Although the system has been driven from its equilibrium state by only an infinitesimal amount, heat has been irreversibly lost due to friction, and cannot be recovered by simply moving the piston infinitesimally in the opposite direction."
See this video link for further details.
- 2
- Link
1
|
Let us start from definitions, as they are given in many sources.
A quasistatic process is an idealized processes that happen out so slowly that a system go through a sequence of states arbitrarily close to equilibrium.
An isobaric process is a process, in which the pressure remains constant.
An isochoric process is a process, in which the volume remains constant.
An isothermal process is a process, in which the temperature remains constant.
Now, I could imagine how an isochoric process could not be quasistatic, but what about isobaric and isothermal processes?
For instance, for the following specific example (from Count Iblis answer):
I indeed object (as Count Iblis expected) that the process of ignition is isothermal: when we ignite the mixture chemical reaction takes place, the huge amount of heat is liberated, and besides, a complicated movement of the gas mixture occurs. Thus, it does not make a lot of sense to speak about keeping the temperature of the system constant, since the temperature is different even in different regions of the system. This should not surprise us, since right after the ignition and for some time then the system is clearly not in equilibrium, and thus, the notion of the temperature of the system is not even defined.
So, if we adopt this definition then, an isothermal process imply a quasistatic one, since it does not make sense to talk about constant temperature in a process that is not quasistatic, since temperature is not even well-defined in intermediate non-equilibrium states of such process.
In other words, the presence of the heat bath is not enough for the process described above to be isothermal: the process should also be performed slowly enough so that all heat liberated can be almostinstantaneously transferred to the surroundings. Only in such a case the temperature of the system is indeed constant during a process. Clearly, the system should be in thermal equilibrium with its surrounding all the way, and thus, I think that for the process to be isothermal, it should be quasistatic.
Am I right?
P.S. Oh, how I feel like I see where I went wrong. An isothermal process indeed must happen at such a slow rate that the thermal equilibrium is maintained, however, thermal equilibrium is not the whole story. As we know systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. So, since only thermal equilibrium is required for an isothermal process, it is not necessarily quasistatic. A quasistatic process is always arbitrarily close to thermodynamic equilibrium, while isothermal is only in thermal equilibrium.
|
Tidak ada komentar:
Posting Komentar